AI-Driven Prenatal Ultrasound System Gets FDA Clearance
Sonio Detect automates exams and detects ultrasound images in real time
At a Glance
- Sonio Detect, an AI-powered ultrasound imaging software, has received FDA clearance.
An AI-powered prenatal ultrasound software developed by a French startup has received FDA clearance.
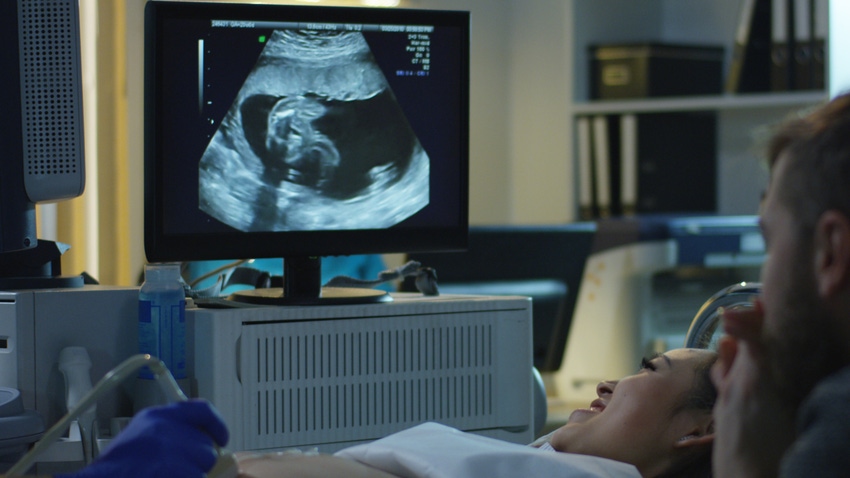
Sonio, headquartered in Paris, developed Sonio Detect, which can automatically detect view and quality criteria of ultrasound images to ensure high quality exams.
Co-founder and CEO Cecile Brosset said in a statement that Sonio Detect is the "first easy to use, manufacturer agnostic ... and efficient quality control solution (that) will lead to better detection of potential anomalies." It works with GE, Samsung and Canon equipment.
In addition, Sonio said it has received $14 million in a funding raise. The investment in addition to the FDA clearance has readied the company to enter the U.S. market.
The prenatal assistant software lets sonographers, maternal-fetal medical professionals, and obstetricians to "raise the bar" in looking for anomalies, the company said.
The product automatically detects views, and anatomical structures within supported views, and confirms quality criteria of the supported views in real time. Health care providers can quickly detect prenatal brain and heart structures, along with other fetal imaging quality criteria, in the womb.
The software has a high accuracy rate in identifying correct labels and views, as well as quality criteria under a wide variety of conditions. It can handle patient BMI, age, ethnicity and gestational ages.
The company validated its system using over 17,000 ultrasound images, showing more than 92% sensitivity on its capability to detect labels and types of ultrasound images. The results showed that Sonio Detect can assist and automatically ensure complete protocols based on images captured by ultrasound technicians.
The AI company has spent over five years researching and collaborating with experts in AI and fetal medicine to develop a prenatal screening and diagnosis tool to increase the accuracy of prenatal imaging results.
Sonio Detect received the FDA's Regulatory Class II 510(k) clearance, meaning it meets regulatory health and safety standards.
Read more about:
Health careAbout the Author(s)
You May Also Like


.jpg?width=700&auto=webp&quality=80&disable=upscale)
.jpg?width=700&auto=webp&quality=80&disable=upscale)

.jpg?width=300&auto=webp&quality=80&disable=upscale)
.jpg?width=300&auto=webp&quality=80&disable=upscale)
.jpg?width=300&auto=webp&quality=80&disable=upscale)
.jpg?width=300&auto=webp&quality=80&disable=upscale)
.jpg?width=300&auto=webp&quality=80&disable=upscale)